
Blood pressure diary (DiGA)
Development of a Digital Health Application (DiGA) as a blood pressure diary for mobile devices (iOS, Android)

Since 2009, we have been supporting our customers in the development of innovative HMI solutions in medical technology. We are also happy to support your software project from the very beginning with the new or further development of desktop, embedded or mobile applications.
In our long-standing projects, we work on equal terms as an extended workbench, process and technology consultant and sparring partner.
To ensure the seamless placing on the market of your medical device, we take into account the following norms and standards. We comply with the European Medical Device Regulation (MDR) and In Vitro Diagnostic (IVDR) regulations.
The German Federal Ministry of Health (BMG) emphasizes the importance of digital health and care applications (DiGA/DiPA) as part of its digitization strategy for public health and care. In addition, the future interoperability requirement with the electronic patient file (ePA) is in focus. We support in the standards-compliant, user-centered development of appropriately secure applications for the cloud through to the integration of edge devices.
A comprehensive quality management system is required for the design and manufacture of medical devices. The focus is on product safety and effectiveness.
Minimum requirements for key software lifecycle processes (development, maintenance, risk management, software configuration management, and software problem resolution).
In order to identify the hazards associated with medical devices, the risks must be assessed, evaluated and regularly controlled.
For so-called 'stand-alone' software, a procedure is suggested here that identifies and manages risks that could arise when using the software.
Medical software is highly complex. But the user interface should not be. Users depend on intuitive operation in their routine situations.
Human-Machine-Interface (HMI) ergonomics guidelines and dialog design principles are important components in user interface design (UI/UX).
When implementing your medical products, we largely dispense with requirement and functional specifications. Instead, we focus on an agile approach so that we can bring your product to market quickly and with high quality. In order to meet the documentation requirements of medical software according to MDR, IVDR, ISO 13485 and IEC 62304, we use Application Lifecycle Management (ALM) systems and/or ticket systems according to defined quality management processes.
Our team is here to accompany you in reaching your goals and turning your visions into reality. With our customized solutions and expertise, we help you enhance your success. Contact us today to learn more. We look forward to hearing from you and assisting you further.

Development of a Digital Health Application (DiGA) as a blood pressure diary for mobile devices (iOS, Android)

Conception and design of a HMI (Human-Machine-Interface) for an AED process serving children and adults.
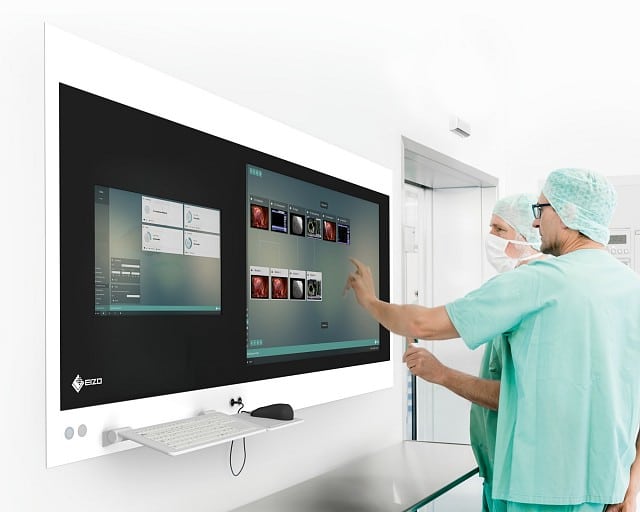
Development of a software for touchless switching of a monitor-layout in operating rooms using hand gestures and voice commands.
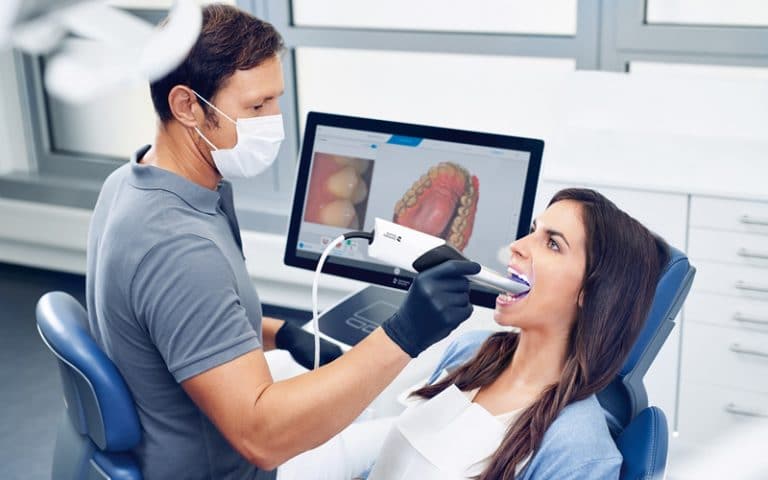
GUI development of a 3D CAD/CAM desktop software for the dental industry.
CONZE Informatik GmbH
Friedrichstrasse 18
57072 Siegen
Germany
+49 (0) 271 240098-50
+49 (0) 271 240098-80
info@conze.com
Business Hours:
Workdays 8 AM - 6 PM
CONZE is bronze sponsor of the German UPA in 2024
Heartfelt wishes and shining children's eyes
Sales Manager Product Distribution
Financial Accounting and Human Resources Officer
GUI Software Developer C++ / Qt
Frontend Software Developer Flutter / Dart